Services for chemical analysis
Our personal and technical equipment allows us to carry out analyses in high quality and under GMP conditions.
Routine analysis
We offer routine analysis by Capillary Electrophoresis for the following analytes:
Analyses according to pharmacopoeia:
Further routine analysis (non-GMP, GMP possible on request):
Download current price list (81 KB): ICA-price list-2023
Download price list galvanics (77 KB): ICA-price-list-galvanics-2023
The determination of all given analytes is normally possible with capillary electrophoresis in aqueous solutions. For other and more complex matrices or specific analytical issues we offer feasibility studies.
In case you are interested in analytes which are not included in the list we gladly prepare an individual offer.
Determination of ionic liquids (IOLI, IL)
New from January 2024:
The CE analyses summarized in the following table can be offered for the IOLI “1-ethyl-3-methylimidazolium tetrachloroaluminate” (EMIM AlCl4). The four CE electrolyte systems can also be supplied.
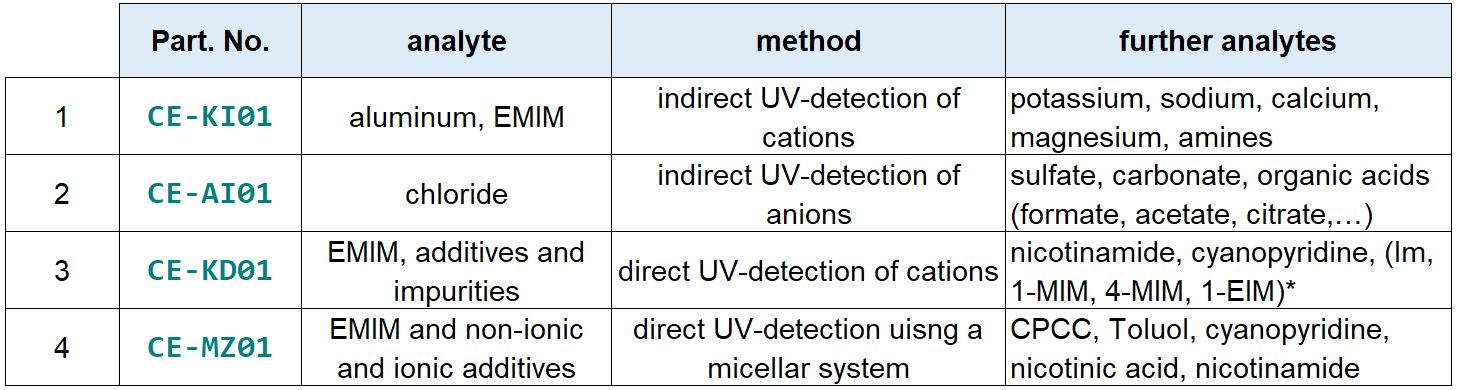
(*Im: imidazole, 1-MIM: 1-methylimidazole, 4-MIM: 4-methylimidazole, 1-EIM: 1-ethylimidazole, CPCC: 2-chloropyridine-3-carbonylchloride)
Download price list CE kits (279 KB): KIT-price-list-2024
Furthermore, we offer the following services:
Scientific Consulting
- Literature research
- Advice on method development (also on-site)
- Expert presentations and trainings
- Creation of trade fair and symposium reports
Method Development
- Assessment of feasibility (feasibility studies)
- Optimization of the methods
- Transfer of methods to different matrices
- Documentation and reporting
- Creation of testing regulations
Method Validation
- Creation of validation plans (for agreed quality characteristics)
- Carrying out the validation
- Creation of the validation report
Carrying out chemical analysis
- Analyses for different areas
- (e.g. environment, food, pharmaceuticals, raw materials, material analysis)
- Creation of analysis plans and reports
- Collaboration with specialized laboratories
Seminars and training
- Our workshop offers
- Organization and implementation of seminars and staff training (also on-site)